Answer:
7.08 grams
Explanations:
Given the reaction below;
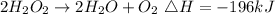
The reaction shows that 2 moles of 2O2 releases 196kJ of energy. Hence 1 moles of H2O2 will. release 98kJ of energy (196/2).
In order to create 20.4kJ of energy, we will need;
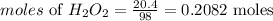
Detemine the mass of H2O2
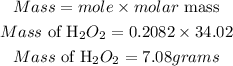
Hence the mass of H2O2 that will react is 7.08 grams