Answer
1. The pH of the solution is 9.69
2. The solution is basic.
Step-by-step explanation
What is given:
The concentration of hydroxide ion, [OH⁻] = 4.91 x 10⁻⁵ M
Step-by-step solution:
1. What is the pH of the solution?
The first approach to this question is to determine the pOH of the hydroxide ion concentration.
The formula to calculate the pOH of a solution is given as:
![pOH=-log[OH^-]](https://img.qammunity.org/2023/formulas/chemistry/college/vqo02fhb0jj8u1geaydn77je0d54m8v5tf.png)
Putting [OH⁻] = 4.91 x 10⁻⁵ into the formula, we have:
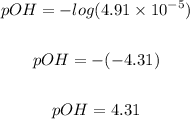
Finally, we can determine the PH of the solution using the relationship between pH and POH, which is:
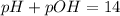
Putting pOH as 4.31, we have the pH of the solution to be:
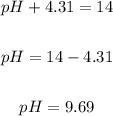
Thus, the pH of the solution is 9.69.
2. Is the solution acidic, basic, or neutral?
Since the pH of the solution is 9.69, then the solution is basic.
pH is a measure of how acidic/basic water is. The range goes from 0 - 14, with 7 being neutral. pHs of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base.