The density of the material is 0.0105g/mL.
1st) We can convert 34.16 inch to cm, knowing that 1 inch is equal to 2.54 cm:
![34.16in\cdot\frac{2.54\operatorname{cm}}{1in}=86.77\operatorname{cm}]()
2nd) Now we can calculate the volume of the cube:
![\begin{gathered} \text{Volume}=(length)^3 \\ \text{Volume}=(86.77cm)^3 \\ \text{Volume}=653,294.2\operatorname{cm}^3 \end{gathered}]()
3rd) It is necessary to convert 6.85kg into g to calculate the density, so:
![6.85\operatorname{kg}\cdot\frac{1000g}{1\operatorname{kg}}=6850g]()
4th) Finally, we can calculate the density of the material using the density formula:
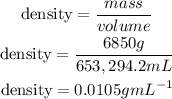
Remember that 1cm3 is equal to 1 mL.
So, the density of the material is 0.0105g/mL.