Answer;
The reaction is endothermic
![C(s)+2S(s)\operatorname{\rightarrow}CS_2(l)\operatorname{\triangle}H=+92kJ]()
Explanations:
The reaction between 1 mol of solid carbon with 2 mol of solid sulfur is given as:
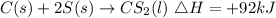
Note that the change in enthalpy is positive since the energy of the product is 92kJ higher than the energy of the reactants. This shows that the enthalpy change of the reaction is greater than zero.
The reaction with a positive enthalpy change is an endothermic reaction showing that heat is absorbed from the surroundings during the reaction.