Given data:
* The mass of the water is 0.65 kg.
* The initial temperature of the water is 20 degree celsius.
* The final temperature of the water is 43 degree celsius.
Solution:
The amount of heat added to the water to increase its temperature is,
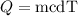
where m is the mass, c is the specific heat, and dT is the change in the temperature,
Substituting the known values,
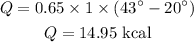
Thus, the amount of heat added to the water is 14.95 kcal or approximately 15 kcal.