Let's see the original equation:
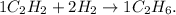
In the reactants, we have 1 mol of C2H2 and 2 moles of H2. And in the products, we have 1 mol of C2H6.
Below the image, you can see that 2 moles of C2H2 are reacting with 8 moles of H2 but you can realize that there is an excess of H2, because if we want to use 2 moles of C2H2, we will require just 4 moles of H2.
You can note this by multiplying each number of moles in the initial equation by two. The chemical reaction, based on these numbers would be:
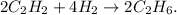
So, in the reaction we're going to have 2 moles of C2H6, on the leftovers reactants: 0 moles of C2H2 and 4 moles of H2 because we're only requiring 4 moles of H2 from 8 moles of this.