Answer: the molality of this solution is 2.00 mol/kg.
Step-by-step explanation:
The question requires us to calculate the molality of a solution prepared with 63.0g of nitric acid and 0.500 kg of water.
The molality of a solution is defined as the amount of solute, in moles, in one kilogram of solvent:
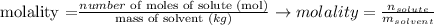
To calculate the molality of the solution presented by the question, we must calculate the amount of moles of HNO3 in 63.0 g of this substance.
The molar mass of HNO3 can be obtained from the atomic masses of hydrogen (H, 1.01 u), nitrogen (N, 14.0 u) and oxygen (O, 16.0 u):
molar mass (HNO3) = (1 * 1.01) + (1 * 14.0) + (3 * 16.0) = 63.0 g/mol
Therefore, knowing the molar mass of HNO3 we can say that there is 1.00 mol of HNO3 in 63.0 g of this compound. Thus, n(solute) = 1.00 mol and m(solvent) = 0.500kg (the mass of water as given by the question), and we can calculate the molality of the solution as:
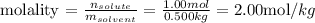
The molality of a solution that contains 63.0g of HNO3 in 0.500kg of water is 2.00 mol/kg (or 2.00 molal).