ANSWER
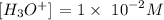
Step-by-step explanation
Given that
The concentration of [OH-] is 1 x 10^-12M
Follow the steps below to find the concentration of [H3O+]
Step 1; Write the pOH formula
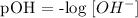
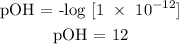
Step 2; Find the pH of the substance
Recall, that pH + pOH = 14
pOH = 12
pH + 12 = 14
subtract 12 from both sides of the equation
pH + 12 - 12 = 14 - 12
pH = 2
Step 3; Find the [H3O+] using the below formula
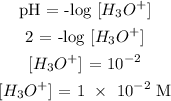
Therefore, the correct answer is option B