Answer:
![7360.775\operatorname{kg}]()
Explanations:
The formula for calculating the density of a substance will be used to get the required mass:
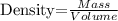
Given the following parameters;
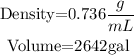
Convert gallons to mL using the conversion rate.
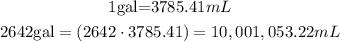
Substitute the given parameters into the formula as shown:
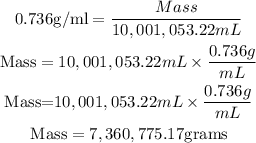
Convert the mass in grams to kilograms
Recall that 1000g = 1kg
![\begin{gathered} \text{Mass=}7,360,775.16grams*\frac{1\operatorname{kg}}{1000\text{grams}} \\ \text{Mass}=\text{=}7,360,775.16\cancel{\text{grams}}*\frac{1\operatorname{kg}}{1000\cancel{\text{grams}}} \\ \text{Mass}=7360.775\operatorname{kg} \end{gathered}]()
Hence the required mass in kg that is in 2642 gal. of a solution whose density you experimentally determined to be 0.736 g/mL is 7360.775kg