Hello there. To solve this question, we simply need to solve this equation for x.
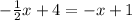
We can start by bringing every term with the variable to the left hand side and other constants to the right hand side.
In order to do so, add x - 4 on both sides of the equation:
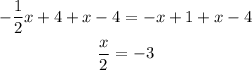
Multiply both sides of the equation by a factor of 2

To make sure this is the right answer, plug it back into the equation you started with:
![undefined]()