Answer:
97.8 g of copper (Cu).
Step-by-step explanation:
What is given?
Volume of hydrogen gas (H2) = 34.5 L.
STP conditions:
Temperature (T) = 273 K.
Pressure (P) = 1 atm.
R = 0.082 L*atm/mol*K.
Step-by-step solution:
Let's find the number of moles of hydrogen gas (H2) to carry out the stoichiometry of the reaction, using the following formula of ideal gas:
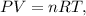
where P is pressure, V is volume, n is the number of moles, R is the ideal gas constant and T is temperature (in scale of Kelvin).
We have to find 'n', so let's solve for this unknown value and replace the given values:
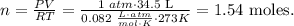
Based on this, we have 1.54 moles of hydrogen gas.
Now, you can see that 1 mol of H2 (hydrogen gas) reacted produces 1 mol of Cu (copper), so the molar ratio between these two compounds is 1:1. This means that 1.54 moles of H2 reacted will produce 1.54 moles of Cu too.
The final step is to do the conversion from 1.54 moles of Cu to mass using the molar mass of Cu which you can find in the periodic table (63.5 g/mol). The conversion would be:
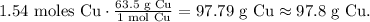
The answer is expected 97.8 g of copper (Cu) using 34.5 L of hydrogen gas at STP conditions.