Answer: A container with 59.6 L would be necessary to hold 2.66 moles of O2 (letter A)
Step-by-step explanation:
The question requires us to determine the volume of 2.66 moles of O2 at STP.
At standard temperature and pressure conditions (STP), 1 mol of any gas corresponds to 22.4 L of the gas. Thus, we can calculate the volume of 2.66 moles of O2 at STP as:
1 mol O2 --------------------- 22.4 L O2
2.66 mol O2 ---------------- x
Solving for x, we'll have:
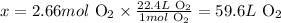
Therefore, 2.66 moles of O2 at STP corresponds to 59.6 L of O2.