Answer:
The ionic equation is the one that expresses both reactants and products as ions when they are dissociated in aqueous solutions.
In this case we have the following chemical reaction:
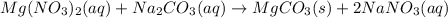
So the ionic equation is the following:
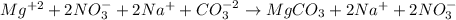
As we can see magnesium carbonate is not an ion in aqueous solution so it is not expressed as one,