We will assume that the gas behaves like an ideal gas. So we can apply the ideal gas law which is described with the following equation:
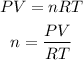
Where,
n is the number of moles of the gas
P is the pressure of the gas. At STP conditions the pressure is 1atm.
T is the temperature of the gas. At STP conditions the temperature is 273.15K
V is the volume of the gas, 0.450L
R is a contant, 0.08206atm.L/mol.K
Now, we replace the known data:
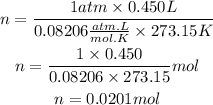
Answer: There are contained 0.0201 mol of argon gas