We have a neutralization reaction between the acid HCl and the base NaOH. The reaction is the following:
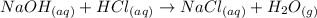
We are asked to find the volume of HCl needed to neutralize a certain volume of NaOH. To find the volume of HCl we will follow the following steps:
1. We calculate the volume of NaOH by subtracting the initial volume minus the final volume.
2. We find the moles of NaOH present in the solution using the molarity term that tells us:
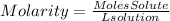
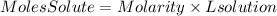
3. By the stoichiometry of the reaction we find the moles of HCl. We have that the ratio HCl to NaOH is 1/1.
4. We find the volume of HCl using the molarity of HCl solution.
Let's proceed with the calculations:
1. Volume of NaOH
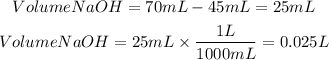
2. Moles of NaOH
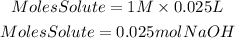
3. Moles HCl
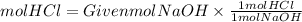
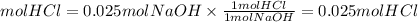
4. Volume of HCl
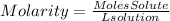
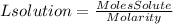
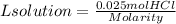
Since we do not have the molarity of the HCl solution, we cannot find the volume of the HCl solution. When the molarity of the HCl solution is known we simply have to replace it in the equation and we will have the volume of the solution in liters.