Since the balanced reaction is:
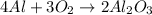
The ratio os O₂ to Al₂O₃ will determine the number of moles produced.
We have excess of Al, so we can only look to O₂ and Al₂O₃.
Their coefficients are 3 for O₂ and 2 for Al₂O₃, so for every 3 moles of O₂, 2 moles of Al₂O₃ will be produced.
Using rules of three, we have:
Al₂O₃ --- O₂
x --- 10 mol
2 mol --- 3 mol
So we have the relation:
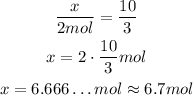
So, 10 mols of O₂ will produce approximately 6.7 mol of Al₂O₃.