ANSWER
option B
Step-by-step explanation
Firstly, we will need to define the standard heat of formation
The standard heat of formation is the amount of heat evolved or absorbed when one mole of a compound is completely formed from its constituent elements under standard conditions.
From the options provided, you will see that only option B aligns with the definition of the standard heat of formation.
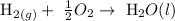
From the above reaction, you will see that 1 mole of water is formed from hydrogen and oxygen.
Hence, the answer is option B