The first step to solve this question is to convert the mass of H2 to moles, using its molecular mass:
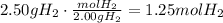
Now, convert the volume of CO to moles using the ideal gas law:

Solve the equation for n and replace P, V, T and R for its known values (1atm, 30.0L, 0.082atmL/molK and 273.15K):

From the given equation we can find how many moles of CO react with 1.25 moles of H2:
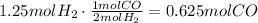
Substract this amount to the value of n to find the excess remaining:
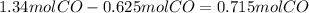
Use the ideal gas law once again, but this time to find the volume occupied by 0.715moles of CO:
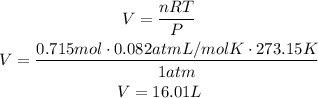
It means that 16.01L of CO are remaining.