Given:
The thermal energy added to the system is Q = 90 J
The work done by the system on the surroundings is W = 30 J
To find the change in internal energy.
Step-by-step explanation:
According to the first law of thermodynamics, the change in internal energy can be calculated by the formula
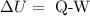
On substituting the values, the change in internal energy will be
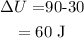
Final Answer: The chage in internal energy is 60 J (option D)