ANSWER
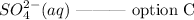
Explanation:
Sulfite ion is readily oxidized to sulfate. This occurs because sulfite ion is exposed to air for a long period of time. Oxidation occurs with atmospheric oxygen.
Below is the oxidation reaction of sulfite ion
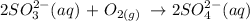
Hence, the possible oxidation product is option C