To solve this question we have to use the ratios of the coefficients of the substances.
For hydrogen gas we have that per one molecule of nitrogen, 3 of hydrogen are used. If 10 molecules of nitrogen are used, then we have that:
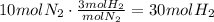
For ammonia gas, per one molecule of nitrogen, 2 of ammonia are produced:
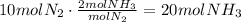
It means that the correct answer is N2=10; H2=30; NH3=20.