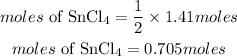Answer:
0.705moles
Explanations:
Given the balanced chemical reaction between Chlorine and SnI4 as shown:
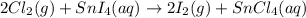
Determine the moles of chlorine gas
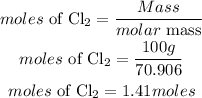
According to stoichometry, 2moles of chlorine gas produces 1 mole of Tin(IV)chloride, the moles of Tin(IV)chloride produced will be: