Answer:
3:2
Explanations:
Before we can determine the mole ratio of compounds in a chemical reaction, the reaction must be balanced first.
Given the balanced chemical reactions;
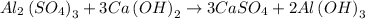
Since the given reaction is balanced, we will determine the number of atom of the calcium hydroxide and aluminium hydroxide in the reaction.
There are 3 moles of calcium hydroxide at the reactant and 2 moles of aluminium hydroxide in the reaction, hence the mole ratio of calcium hydroxide to aluminum hydroxide is 3:2