Answer:
Amount of heat lost by the copper block = - 3319.59 J.
Amount of heat gained by the water = 3260.4 J.
Step-by-step explanation:
What is given?
Mass of water = 150.0 g.
Initial temperature of water = 25.1 °C
Mass of copper = 123.0 g.
Initial temperature of copper = 100.4 °C.
Final temperature (general) = 30.3 °C.
Specific heat of water = 4.18 J/gK.
Specific heat of copper = 0.385 J/gK.
Step-by-step solution:
First, let's find the amount of heat lost in J of the copper block.
The formula of heat is the following:
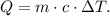
Where Q is heat, m is the mass, c is the specific heat, and ΔT is the change of temperature. Remember that ΔT is: ΔT = Final temperature - Initial temperature.
*The specific heat has the units of J/gK and J/g°C but both are numerically equal.
Now, let's replace the given data of the copper block in the formula, like this:
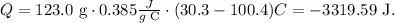
The amount of heat lost by the copper block is -3319.59 J.
Now, let's calculate the amount of heat gained by water following the same process that we did before but with the data that we have of water, like this:
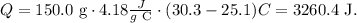
The amount of heat gained by the water is 3260.4 J.