Chemistry => Solutions => Dilution
A solution is a mixture of two or more substances. In this case, we have a solution of acid in water. For dilutions we can apply the following equation:
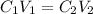
Where,
C1 is the initial concentration, 45%
V1 is the initial volume of the solution, 70mL
C2 is the final concentration, 30%
V2 is the final volume of the solution, unknown
Now, we clear V2 and replace the known data:
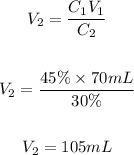
The final volume of the solution will be 105 mL. The water should be added will be:
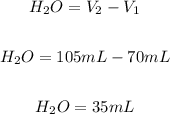
Answer: To dilute the solution from 45% to 30%, should be added 35 mL of water.