We have
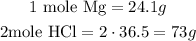
So, we have that 24.1g Mg reacts with 73g HCl.
Then,
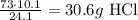
Taking into account that 26g HCl was added to the reaction. We say that HCl is the limiting reagent.
The limiting reagent is the reagent that determines how much product is going to be obtained. We can see that the HCl is present in less quantity, so HCl determines when the reaction finish because it is the reagent that will run out first.