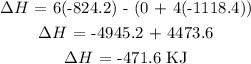Answer:
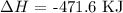
Step-by-step explanation:
Here, we want to get the enthalpy of reaction for the given reaction
In order to get this, we shall be using the heat of the formation of the products and reactants
For a molecule like oxygen that contains an atom of the same element, the heat of formation is written as zero
The enthalpy of reaction can be written as follows:
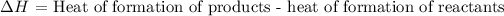
The heat of formation of magenetite Fe3O4 is -1118.4 KJ/mol
The heat of formation of Fe2O3 is -824.2 KJ/mol
The enthalpy of reaction is thus: