Given:
The function is s(t)=0.2t+14
The residual is calculated by,
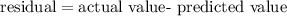
Let's calculate the values for function s(t) at t=0,1,2,3,4,5,6,7,8,9.
For t=0,
s(t)=0.2(t)+14
s(0)=0.2(0)+14=14
And residual If you don’t need further explanation on this question, we can end the session. I’d really appreciate you letting me know how I did by rating our session after you exit. Thanks and have a great day!for t=0,
Residual = actual value- predicted value
Residual=14.4-14=0.4