Answer
The percent yield for the reaction is 106.77%
Step-by-step explanation
Given:
Moles of Na = 2.42 mol
Actual yield of table salt (NaCl) produced = 151 g
What to find:
The percent yield for the reaction.
Step-by-step solution:
The first step is to write a balanced equation for the reaction.
2Na + Cl₂ → 2NaCl
From the equation,
2 moles of Na produced 2 moles of NaCl
So, 2.42 moles of Na will produce x moles of NaCl
The value of x is calculated below
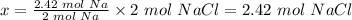
The next step is to calculate the theoretical yield of NaCl.
1 mole NaCl = 58.44 grams
2.42 moles NaCl = x grams
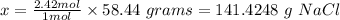
The final step is to calculate the percent yield using the formula below:
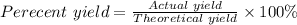
Actual yield = 151 g
Theoretical yield = 141.4248 g
Thus,
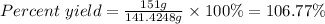
The percent yield for the reaction is 106.77%