Answer
We have the following chemical reaction:
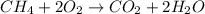
Now we calculate how many moles (n) of CH4 react, we use its molar mass:
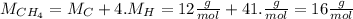
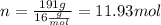
Now we calculate the moles of CO2 produced. As we can see from the equation for every mol of CH4 that reacts 1 mol of CO2 is produced. So in this case 11.93 moles are produced. Now we calculate the corrisponding mass.
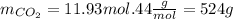
So the answer is 524g.