Answer:
d) 467.52grams
e) 291.664grams
f) 238.286gram
Explanations:
The reaction between sodium carbonate and hydrochloric acid is given as:
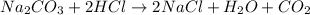
a) From the given reactant, the limiting reactant will have less number of moles while the excess reactant will have higher number of moles.
Since the limiting reactant will be HCl (8moles/2 = 4moles per at)mi
b) Te excess rectant will be Na2CO3
c) According to the reactant, 8moles of NaCl will b produced since t2 moles of HCl produces 2 moles of Nacl
d) Since NaCl is the limiting reactant, the mass of NaC will be calculated as:
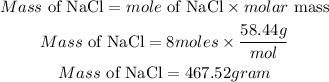
e) Since the limiting reactant is HCl (mass that was used up), the mass of HCl will be given as:
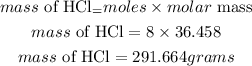
Hence the mass of excess reactant that react and get used up is 291.664 grams
f) Mass of excess reactant that remain = Mass of excess reactant - Mass of limiting reactant
Determine the mass of exctss reactant
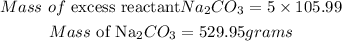
Mass of reactant that remain = 529.95 - 291.664 = 238.286grams
Hence the grams of excess reactant that remains is 238.286grams