Answer
V₂ = 0.0727 L or 72.7 mL
Step-by-step explanation
The general gas law is given by:
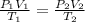
To find V₂
V₁ = 56.7 mL = 56.7/1000 = 0.0567 L
T₁ = -34.0 °C = -34.0 + 273 = 239 K
P₁ = 1.07 atm
T₂ = 375 K
P₂ = 998 torr = 998 x 0.00131579 = 1.31 atm
From the combine gas law, V₂ is
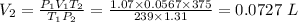
V₂ = 0.0727 L or 72.7 mL