We need to convert 1.2x10^21 formula units of magnesium hydroxide to grams
First, we must use that
Using that, we can calculate the number of moles of magnesium hydroxide
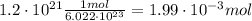
Then, using that the molar mass of magnesium hydroxide is 58 g/mol we can calculate the grams
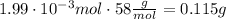
Finally, 1.2x10^21 formula units of magnesium hydroxide are 0.115 grams
ANSWER:
0.115