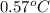Given
Solar radiation at the top of the atmosphere,
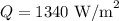
Solar radiation at the bottom of the atmosphere,
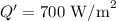
Solar radiation absorbed by the atmosphere is
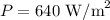
The time, t=24 hr=24x60x60 s
Specific heat,
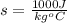
The radius of the earth,
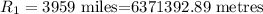
The length with the atmosphere,
To find
Determine how much the temperature would increase in the atmosphere by the absorption of the solar radiation
Step-by-step explanation
Volume of the atmosphere,
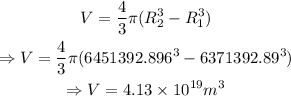
Now,
Mass
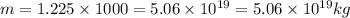
Thus the change in temperature is
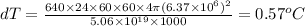
Conclusion
The change in temperature is