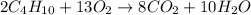
We will firstly convert the mass into moles:
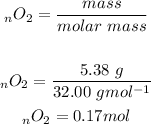
Based on the mole ratio, 13 moles of oxygen gas produces 10 moles of water molecules. Based on this relationship we can determine the moles of water molecules produce if we reacted 0.17 mole of oxygen gas:
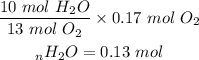
Now we would convert this mole to mass:
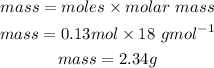
Answer: 2.34g of water is produced from 5.38g of oxygen.