Answer:
60.48%
Step-by-step explanation
The combustion of propane is expressed as:
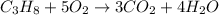
Determine the moles of water
Moles of water = 68.3/18
Moles of water = 3.79moles
Based on stoichiometry, 1 moles of propane produce 4 moles of water, the moles of propane required will be:
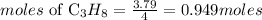
Determine the mass of propane (theoretical yield)
Mass of propane = 0.949 * 44.1
Mass of propane = 41.83grams
Determine the percentage yield

Hence the percent yield is 60.48%