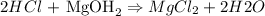Answer:Molarity of (MOM ) = 0.048 M
Explanation :
In this reaction , HCL acts as an acid and MOM act as a base
Given :
• Acid Concentration HCL = 0.2 M
,
• Average volume of HCl = 24 mL
,
• Volume Base (MOM) = 100 mL
,
• Concentration (MOM) = ?M
We will calculate the concentration of MOM by applying the dilutions formula :
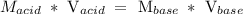
Substituting the given parameters into the formula above, we calculate that Molarity (concentration) of MOM is :
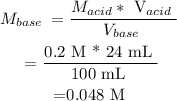
This means that Molarity of (MOM ) = 0.048 M
Post lab question 1 :
Balanced reaction as follows :