To answer this, we will need:
1: convert the mass of HCl to number of moles of HCl;
2: make the stoichimetry to see the corresponding number of moles of CO₂;
3: convert the number of moles of CO₂ to mass.
To convert the mass to number of moles, we will use:
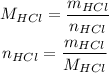
The molar mass of HCl can be calculated using the molar masses of its atoms:
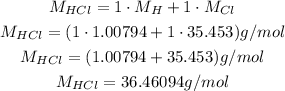
So:
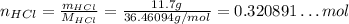
Now, the ratio of the number of moles of the components of a reaction has to obey the stoichiometry.
Accouding to the reaction equation, 2 moles of HCl produce 1 mol of CO₂.
Thus, if x is the number of moles of CO₂ produced:
x --- 1 mol CO₂
0.320891... mol HCl --- 2 mol HCl

Now, we just need to convert the number of moles to mass:
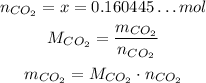
The molar mass of CO₂ is:
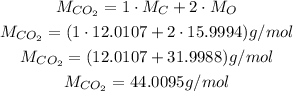
So:
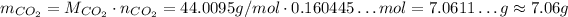
Thus, assuming all the HCl react, approximately 7.06 g of CO₂ will be produced.