ANSWER
The number of moles of methane is 905.32 moles
Explanation:
Given information
The number of particles of methane = 5.45 x 10^26 particles
Let x represents the number of moles of methane
To calculate the number of moles, we will be using the below formula
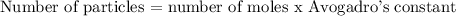
Recall that, the Avogadro's constant is given as
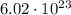
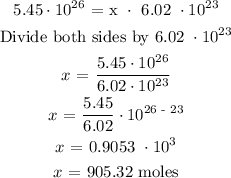
Therefore, the number of moles of methane is 905.32 moles