To verify if an equation is balanced, you have the count each one of the present atoms on both sides of it. Let's count on each one of the options until finding the one that's balanced:
A.
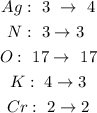
The Ag and the K are not balanced.
B.
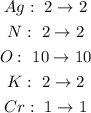
It means that this equation is balanced.
The answer is B.