Given:
The mass of water, m=15 g=0.015 kg
The initial temperature of the water, T₁=50 °C
The final temperature of the ater, T ₂=100 °C
To find:
The heat required.
Step-by-step explanation:
The specific heat of water is, c=4180 J/(kg K)
The heat required to raise the temperature of the water is given by,
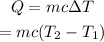
On substituting the known values,
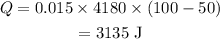
Final answer:
Thus the correct answer is option C.