The first step we have to follow is to convert the given tons to grams:
![8.0*10^4tons\cdot\frac{1000\operatorname{kg}}{1ton}\cdot\frac{1000g}{1\operatorname{kg}}=8*10^(10)g]()
Use the given ratio of grams of Mg to kg of seawater to find how much of seawater is needed:
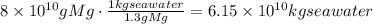
Use the density of seawater to find how many liters are needed:
![6.15*10^(10)\operatorname{kg}\cdot\frac{1000g}{1\operatorname{kg}}\cdot(1ml)/(1.03g)\cdot(1l)/(1000ml)=5.97*10^(10)l]()
5.97x10^10 liters are needed to extract that amount of Mg.