The proportion of number to mol is 1 mol to approximately 6.022 x 10^23, that is, there are 6.022 x 10^23 of somthing in 1 mol of it.
So, using rule of three, we have:
2.8 x 10^23 molecules --- x
6.022 x 10^23 molecules --- 1 mol
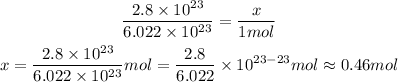
So, this is approximately 0.46 mol or 4.6 x 10^-1 mol.