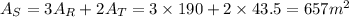To calculate the volume of this prism, we could just find the area of the surface of the triangle, and multiply by the lenght.
The area of the triangle is given by the product of height by the base, divided by 2:
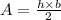
Since we have the following data:
height = 8.7m
base of the triangle = 10m
lenght = 19m
The area of the base of this prism is:
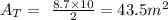
Multiplying it by the lenght we get the volume of the prism:
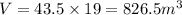
Now, to calculate the area of the surface, we can calculate the area of each face then add them up. We already calculate the area of the triangular bases, we just need to calculate the area of the rectangular faces.
The rectangular face area is given as the product of the lenght by the side of the base, then the area of the rectangular face is:
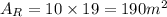
Since we have 3 rectangular faces and two triagular basis, the surface area is given by: