A solution of KOH solution was made, and we are asked to find the volume of the initial solution. We will apply the following equation to find it:
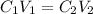
C1 is the concentration of the original solution, 2.25M
V1 is the volume we have to find
C2 is the final concentration, 0.60M
V2 is the final concentration 120mL
We clear V1 and replace the known data:
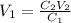
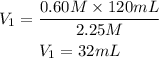
The volume of the original sample is 32mL
Answer: 32mL