Chemistry => Stoichiometry => Mole Ratios
The molar ratio is equal to the relationship in moles between different compounds.
They give us the balanced equation of the reaction, meaning that the atoms in the reactants have the same number in the products. 6 hydrogen atoms and 2 nitrogen atoms on each side of the reaction.
To find the moles of nitrogen that are required we will find the molar ratio between nitrogen and hydrogen, for them, we divide the stoichiometric coefficients of the balanced equation of the reaction.
So, the ratio Nitrogen to Hydrogen will be 1/3.
The moles of nitrogen that react with 18 mol of hydrogen will be:
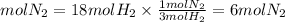
Answer: a. 6 mol