To solve this problem, we have to use Dalton's Law about partial pressure.
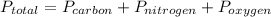
However, we have to use the following formula to find each pressure.
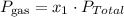
Where x refers to the fraction molar relation between the moles of the whole container and the moles of each gas.
Let's apply the formula to each gas
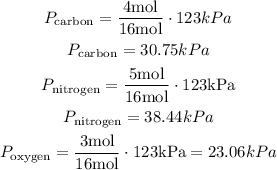
Therefore, the partial pressure of the oxygen is 23.06 kPa, approximately.