Answer:
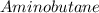
Step-by-step explanation:
Here, we want to get the functional group that the compound belongs to
As we can see, there is a carbon chain that ends with NH2
R-NH2 here is termed the amine group (where R is an alkyl or aryl group)
The R we have here which is the principal carbon chain has 4 carbon atoms
That is an indication of a but- prefix
Thus, we have a but and an amine
This would give the name of the compound as Aminobutane (which in IUPAC nomenclature is 1-aminobutane)