In order to calculate the amount of energy required to increase the temperature of both boiler and water to 80°C, use the following expression:
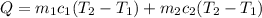
Q is the required heat to increase the temperature of boiler and water. As you can notice, is the sum of the heat required to increase the temperature of boiler and water separately.
m1: mass of boiler = 520kg
m2: water mass = 110 kg
c1: specific heat of water = 4182 J/kg*C
c2: specific heat of steel = 420 J/kg*C (boiler)
T1: intial temperature = 40°C
T2: final temperature = 80°C
Replace the previous values of the parameters into the formula for Q and simplify:
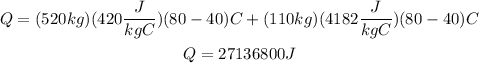
Now, consider that 1kCal is:
1 kCal = 4184J
Then, you obtain for Q:
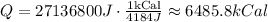
Hence, approximately 6485.8kCal is required to increase the temperature of both boiler and water to 80°C