Answer:
44.4 moles of NH3.
Step-by-step explanation:
You can see that in the chemical equation, we have that 4 moles of NH3 reacted produces 6 moles of H2O. We can state the rule of three to find how many moles of NH3 we need to produce 66.6 moles of H2O, like this:
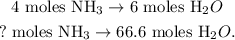
The calculation would be:
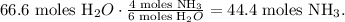
We will need 44.4 moles of NH3 to produce 66.6 moles of H2O.